iPS Cells

iPS Cells facility is located in the Arvo building on the Kauppi campus of the University of Tampere. The facility provides services related to iPS cell technology for academic and non-academic clients.
Introduction
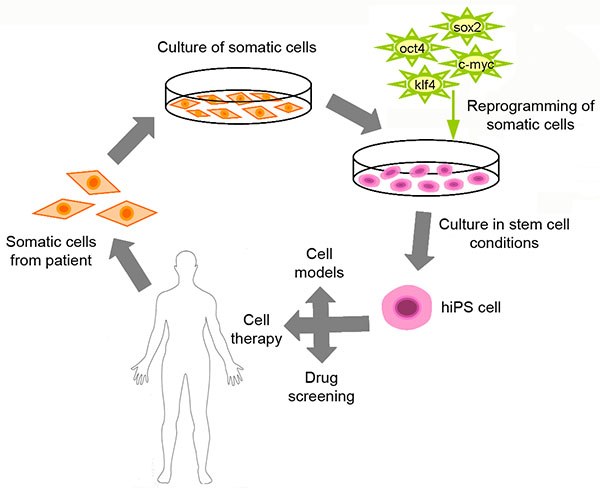
Already fully differentiated adult cells can be reprogrammed into pluripotent cells in defined conditions. Reprogrammed cells are called induced pluripotent stem cells, iPS cells, and they can be differentiated into desired cell type, retaining the original genotype. With this technology, it is possible to create patient specific stem cell lines, which provide a way to model and study the pathophysiology of various disorders in human cells. Therefore, iPS cell technology offers a promising and safe platform to screen and optimize patient specific drug therapy.
iPS Cells facility is located in the Arvo building on the Kauppi campus of the Tampere University. The facility provides services related to iPS cell technology for academic and non-academic clients.
ACKNOWLEDGEMENT
All the users of the iPS Cells facility services are obligated to acknowledge the facility and Biocenter Finland (BF) in publications:
“The authors acknowledge the Biocenter Finland and Tampere facility of iPS Cells for the service.”
This facility is partly supported by the Biocenter Finland technology platform network.

Methods
We have optimized both integrative as well as non-integrative reprogramming methods at the iPS Cells facility. iPS cells can be reprogrammed using either retroviruses or non-viral methods (e.g. episomal vectors). Reprogramming has been optimized for fibroblasts.
The iPS cells have the capacity to differentiate into derivatives from all three germ layers. In defined conditions iPS cells can be differentiated into the cell type of interest including cardiomyocytes, neural cells and hepatocytes.
iPS Cells facility has established novel assays to automatically or semi-automatically analyze the morphology and functionality of cardiomyocytes. The assays include analysis of cell size and orientation, as well as detailed analysis of Ca2+ transients, electrical and beating behavior of the cells. Besides cardiomyocytes, these assays can be applied on many other cell types.
Services
iPS Cells facility provides following services:
- Taking a skin biopsy
- Provision of human iPS cells
- Large collection of disease specific iPS cells, as well as iPS cells derived from healthy control individuals
- Generation of human iPS cells from fibroblasts provided by client
- A supportive statement from the ethical committee is required
- Generation and basic characterization of at least two human iPS cell clones
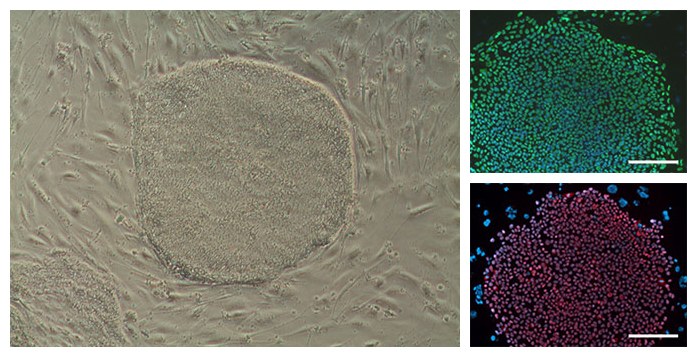
- Characterization of human iPS cells
- pluripotency marker characterization (Nanog, Oct4, TRA-1-60, TRA-1-81)
- PCR analysis of endogenous and exogenous markers
- In vitro pluripotency analysis by EB formation
- karyotype analysis (service bought from outside)
- Differentiation of human iPS cells upon request
- cardiomyocytes, hepatocytes
- Provision of differentiated cells
- cardiomyocytes, hepatocytes
- Characterization of differentiated cells
- Optimized assays for studying the morphology (cell size and orientation) and functionality (Ca2+ transients and basic electrophysiology), as well as beating behavior (video analysis) of differentiated cells
- Assays have been optimized for the iPS-derived cardiomyocytes but same methods can be applied for other cell types
- Rent of special equipment
- 4D-Nucleofector™ System
- EVOS™ FL Cell Imaging System (light cubes: DAPI, GFP, RFP)
- Hands-on training
- iPS Cells facility provides customized hands-on training for clients
Prices
We make iPS cell lines mainly from PBMCs utilizing Nucleofection and plasmid vectors. Pricing is heavily dependent on the project details and how long the newly produced iPS cell lines are cultured and whether any characterizations are done. Please, contact for details and up-to-date price information and for non-academic prices. Below you can find an estimate for iPS cell line generation and characterization prices (as of 01/2023).
|
Product |
Academic price* |
|
iPS cell line generation from PBMC** |
700 € |
|
Characterization of iPSC lines*** |
|
|
one cell line |
1 400 € |
|
two cell lines from same patient |
2 700 € |
|
Differentiation of iPS cells Cardiomyocytes |
upon request |
|
Analysis of differentiated cells |
upon request |
|
cell size and orientation Ca2+ transients basic electrophysiology mechanical movement |
|
|
Hands-on training |
upon request |
|
Rent of Nucleofector and EVOS microscopes |
upon request |
* prices are subject to change, please contact for up-to-date prices **includes isolation and nucleofection of PBMCs, colony picking and cell culture until p4 (no characterization). *** includes cell culture until p10 and characterization assays (details available on request)
Publications
2022
- Häkli, M, Kreutzer, J, Mäki, AJ, Välimäki, H, Cherian, RM, Kallio, P et al.. Electrophysiological Changes of Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes during Acute Hypoxia and Reoxygenation. Stem Cells Int. 2022;2022 :9438281. doi: 10.1155/2022/9438281. PubMed PMID:36579142 PubMed Central PMC9792238.
- Le Dour, C, Chatzifrangkeskou, M, Macquart, C, Magiera, MM, Peccate, C, Jouve, C et al.. Actin-microtubule cytoskeletal interplay mediated by MRTF-A/SRF signaling promotes dilated cardiomyopathy caused by LMNA mutations. Nat Commun. 2022;13 (1):7886. doi: 10.1038/s41467-022-35639-x. PubMed PMID:36550158 PubMed Central PMC9780334.
- Prajapati, C, Koivumäki, J, Pekkanen-Mattila, M, Aalto-Setälä, K. Sex differences in heart: from basics to clinics. Eur J Med Res. 2022;27 (1):241. doi: 10.1186/s40001-022-00880-z. PubMed PMID:36352432PubMed Central PMC9647968.
- Rodosthenous, RS, Niemi, MEK, Kallio, L, Perala, M, Terho, P, Knopp, T et al.. Recontacting biobank participants to collect lifestyle, behavioural and cognitive information via online questionnaires: lessons from a pilot study within FinnGen. BMJ Open. 2022;12 (10):e064695. doi: 10.1136/bmjopen-2022-064695. PubMed PMID:36198465 PubMed Central PMC9535212.
- Maria Cherian, R, Prajapati, C, Penttinen, K, Häkli, M, Koivisto, JT, Pekkanen-Mattila, M et al.. Fluorescent hiPSC-derived MYH6-mScarlet cardiomyocytes for real-time tracking, imaging, and cardiotoxicity assays. Cell Biol Toxicol. 2022; :. doi: 10.1007/s10565-022-09742-0. PubMed PMID:35870039 .
- Häkli, M, Jäntti, S, Joki, T, Sukki, L, Tornberg, K, Aalto-Setälä, K et al.. Human Neurons Form Axon-Mediated Functional Connections with Human Cardiomyocytes in Compartmentalized Microfluidic Chip. Int J Mol Sci. 2022;23 (6):. doi: 10.3390/ijms23063148. PubMed PMID:35328569 PubMed Central PMC8955890.
- Gaballah, M, Penttinen, K, Kreutzer, J, Mäki, AJ, Kallio, P, Aalto-Setälä, K et al.. Cardiac Ischemia On-a-Chip: Antiarrhythmic Effect of Levosimendan on Ischemic Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Cells. 2022;11 (6):. doi: 10.3390/cells11061045. PubMed PMID:35326497 PubMed Central PMC8947267.
- Tamlander, M, Mars, N, Pirinen, M, FinnGen, Widén, E, Ripatti, S et al.. Integration of questionnaire-based risk factors improves polygenic risk scores for human coronary heart disease and type 2 diabetes. Commun Biol. 2022;5 (1):158. doi: 10.1038/s42003-021-02996-0. PubMed PMID:35197564 PubMed Central PMC8866413.
- Saari, J, Siddique, F, Korpela, S, Mäntylä, E, Ihalainen, TO, Kaukinen, K et al.. Toward Xeno-Free Differentiation of Human Induced Pluripotent Stem Cell-Derived Small Intestinal Epithelial Cells. Int J Mol Sci. 2022;23 (3):. doi: 10.3390/ijms23031312. PubMed PMID:35163236 PubMed Central PMC8835723.
- Juhola, M, Joutsijoki, H, Penttinen, K, Shah, D, Pölönen, RP, Aalto-Setälä, K et al.. Data analytics for cardiac diseases. Comput Biol Med. 2022;142 :105218. doi: 10.1016/j.compbiomed.2022.105218. PubMed PMID:34999413 .
2021
- Juhola, M, Joutsijoki, H, Penttinen, K, Shah, D, Aalto-Setälä, K. On computational classification of genetic cardiac diseases applying iPSC cardiomyocytes. Comput Methods Programs Biomed. 2021;210 :106367. doi: 10.1016/j.cmpb.2021.106367. PubMed PMID:34474196 .
- Marttila, S, Viiri, LE, Mishra, PP, Kühnel, B, Matias-Garcia, PR, Lyytikäinen, LP et al.. Methylation status of nc886 epiallele reflects periconceptional conditions and is associated with glucose metabolism through nc886 RNAs. Clin Epigenetics. 2021;13 (1):143. doi: 10.1186/s13148-021-01132-3. PubMed PMID:34294131 PubMed Central PMC8296652.
- Häkli, M, Kreutzer, J, Mäki, AJ, Välimäki, H, Lappi, H, Huhtala, H et al.. Human induced pluripotent stem cell-based platform for modeling cardiac ischemia. Sci Rep. 2021;11 (1):4153. doi: 10.1038/s41598-021-83740-w. PubMed PMID:33603154 PubMed Central PMC7893031.
- Prajapati, C, Ojala, M, Lappi, H, Aalto-Setälä, K, Pekkanen-Mattila, M. Electrophysiological evaluation of human induced pluripotent stem cell-derived cardiomyocytes obtained by different methods. Stem Cell Res. 2021;51 :102176. doi: 10.1016/j.scr.2021.102176. PubMed PMID:33485184 .
- Juhola, M, Penttinen, K, Joutsijoki, H, Aalto-Setälä, K. Analysis of Drug Effects on iPSC Cardiomyocytes with Machine Learning. Ann Biomed Eng. 2021;49 (1):129-138. doi: 10.1007/s10439-020-02521-0. PubMed PMID:32367466 PubMed Central PMC7773623.
2020
- Zelnik, ID, Volpert, G, Viiri, LE, Kauhanen, D, Arazi, T, Aalto-Setälä, K et al.. Different rates of flux through the biosynthetic pathway for long-chain versus very-long-chain sphingolipids. J Lipid Res. 2020;61 (10):1341-1346. doi: 10.1194/jlr.RA120000984. PubMed PMID:32651186 PubMed Central PMC7529049.
- Välimäki, H, Hyvärinen, T, Leivo, J, Iftikhar, H, Pekkanen-Mattila, M, Rajan, DK et al.. Covalent immobilization of luminescent oxygen indicators reduces cytotoxicity. Biomed Microdevices. 2020;22 (2):41. doi: 10.1007/s10544-020-00495-3. PubMed PMID:32494857 PubMed Central PMC7270993.
- Shah, D, Prajapati, C, Penttinen, K, Cherian, RM, Koivumäki, JT, Alexanova, A et al.. hiPSC-Derived Cardiomyocyte Model of LQT2 Syndrome Derived from Asymptomatic and Symptomatic Mutation Carriers Reproduces Clinical Differences in Aggregates but Not in Single Cells. Cells. 2020;9 (5):. doi: 10.3390/cells9051153. PubMed PMID:32392813 PubMed Central PMC7290503.
- Juhola, M, Penttinen, K, Joutsijoki, H, Aalto-Setälä, K. Analysis of Drug Effects on iPSC Cardiomyocytes with Machine Learning. Ann Biomed Eng. 2021;49 (1):129-138. doi: 10.1007/s10439-020-02521-0. PubMed PMID:32367466 PubMed Central PMC7773623.
- Heliö, K, Kangas-Kontio, T, Weckström, S, Vanninen, SUM, Aalto-Setälä, K, Alastalo, TP et al.. DSP p.(Thr2104Glnfs*12) variant presents variably with early onset severe arrhythmias and left ventricular cardiomyopathy. BMC Med Genet. 2020;21 (1):19. doi: 10.1186/s12881-020-0955-z. PubMed PMID:32005173 PubMed Central PMC6995042.
- Calejo, MT, Saari, J, Vuorenpää, H, Vuorimaa-Laukkanen, E, Kallio, P, Aalto-Setälä, K et al.. Co-culture of human induced pluripotent stem cell-derived retinal pigment epithelial cells and endothelial cells on double collagen-coated honeycomb films. Acta Biomater. 2020;101 :327-343. doi: 10.1016/j.actbio.2019.11.002. PubMed PMID:31711900 .
- Kreutzer, J, Viehrig, M, Pölönen, RP, Zhao, F, Ojala, M, Aalto-Setälä, K et al.. Pneumatic unidirectional cell stretching device for mechanobiological studies of cardiomyocytes. Biomech Model Mechanobiol. 2020;19 (1):291-303. doi: 10.1007/s10237-019-01211-8. PubMed PMID:31444593 PubMed Central PMC7005075.
2019
- Joutsijoki, H, Penttinen, K, Juhola, M, Aalto-Setälä, K. Separation of HCM and LQT Cardiac Diseases with Machine Learning of Ca2+ Transient Profiles. Methods Inf Med. 2019;58 (4-05):167-178. doi: 10.1055/s-0040-1701484. PubMed PMID:32079026 .
- Calejo, MT, Saari, J, Vuorenpää, H, Vuorimaa-Laukkanen, E, Kallio, P, Aalto-Setälä, K et al.. Co-culture of human induced pluripotent stem cell-derived retinal pigment epithelial cells and endothelial cells on double collagen-coated honeycomb films. Acta Biomater. 2020;101 :327-343. doi: 10.1016/j.actbio.2019.11.002. PubMed PMID:31711900 .
- Kreutzer, J, Viehrig, M, Pölönen, RP, Zhao, F, Ojala, M, Aalto-Setälä, K et al.. Pneumatic unidirectional cell stretching device for mechanobiological studies of cardiomyocytes. Biomech Model Mechanobiol. 2020;19 (1):291-303. doi: 10.1007/s10237-019-01211-8. PubMed PMID:31444593 PubMed Central PMC7005075.
- Kiamehr, M, Klettner, A, Richert, E, Koskela, A, Koistinen, A, Skottman, H et al.. Compromised Barrier Function in Human Induced Pluripotent Stem-Cell-Derived Retinal Pigment Epithelial Cells from Type 2 Diabetic Patients. Int J Mol Sci. 2019;20 (15):. doi: 10.3390/ijms20153773. PubMed PMID:31375001PubMed Central PMC6696227.
- Shah, D, Virtanen, L, Prajapati, C, Kiamehr, M, Gullmets, J, West, G et al.. Modeling of LMNA-Related Dilated Cardiomyopathy Using Human Induced Pluripotent Stem Cells. Cells. 2019;8 (6):. doi: 10.3390/cells8060594. PubMed PMID:31208058 PubMed Central PMC6627421.
- Kiamehr, M, Heiskanen, L, Laufer, T, Düsterloh, A, Kahraman, M, Käkelä, R et al.. Dedifferentiation of Primary Hepatocytes is Accompanied with Reorganization of Lipid Metabolism Indicated by Altered Molecular Lipid and miRNA Profiles. Int J Mol Sci. 2019;20 (12):. doi: 10.3390/ijms20122910. PubMed PMID:31207892 PubMed Central PMC6627955.
- Pekkanen-Mattila, M, Häkli, M, Pölönen, RP, Mansikkala, T, Junnila, A, Talvitie, E et al.. Polyethylene Terephthalate Textiles Enhance the Structural Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Materials (Basel). 2019;12 (11):. doi: 10.3390/ma12111805. PubMed PMID:31163704 PubMed Central PMC6600740.
- Koivisto, JT, Gering, C, Karvinen, J, Maria Cherian, R, Belay, B, Hyttinen, J et al.. Mechanically Biomimetic Gelatin-Gellan Gum Hydrogels for 3D Culture of Beating Human Cardiomyocytes. ACS Appl Mater Interfaces. 2019;11 (23):20589-20602. doi: 10.1021/acsami.8b22343. PubMed PMID:31120238PubMed Central PMC6750838.
- Kim, J, Shah, D, Potapov, I, Latukka, J, Aalto-Setälä, K, Räsänen, E et al.. Scaling and correlation properties of RR and QT intervals at the cellular level. Sci Rep. 2019;9 (1):3651. doi: 10.1038/s41598-019-40247-9. PubMed PMID:30842620 PubMed Central PMC6403385.
- Viiri, LE, Rantapero, T, Kiamehr, M, Alexanova, A, Oittinen, M, Viiri, K et al.. Extensive reprogramming of the nascent transcriptome during iPSC to hepatocyte differentiation. Sci Rep. 2019;9 (1):3562. doi: 10.1038/s41598-019-39215-0. PubMed PMID:30837492 PubMed Central PMC6401154.
- Jääskeläinen, P, Vangipurapu, J, Raivo, J, Kuulasmaa, T, Heliö, T, Aalto-Setälä, K et al.. Genetic basis and outcome in a nationwide study of Finnish patients with hypertrophic cardiomyopathy. ESC Heart Fail. 2019;6 (2):436-445. doi: 10.1002/ehf2.12420. PubMed PMID:30775854 PubMed Central PMC6437444.
- Kiamehr, M, Alexanova, A, Viiri, LE, Heiskanen, L, Vihervaara, T, Kauhanen, D et al.. hiPSC-derived hepatocytes closely mimic the lipid profile of primary hepatocytes: A future personalised cell model for studying the lipid metabolism of the liver. J Cell Physiol. 2019;234 (4):3744-3761. doi: 10.1002/jcp.27131. PubMed PMID:30146765 .
2018
- Potapov, I, Latukka, J, Kim, J, Luukko, P, Aalto-Setälä, K, Räsänen, E et al.. Information transfer in QT-RR dynamics: Application to QT-correction. Sci Rep. 2018;8 (1):14992. doi: 10.1038/s41598-018-33359-1. PubMed PMID:30301929 PubMed Central PMC6178346.
- Vanninen, SUM, Leivo, K, Seppälä, EH, Aalto-Setälä, K, Pitkänen, O, Suursalmi, P et al.. Heterozygous junctophilin-2 (JPH2) p.(Thr161Lys) is a monogenic cause for HCM with heart failure. PLoS One. 2018;13 (9):e0203422. doi: 10.1371/journal.pone.0203422. PubMed PMID:30235249 PubMed Central PMC6147424.
- Kiamehr, M, Alexanova, A, Viiri, LE, Heiskanen, L, Vihervaara, T, Kauhanen, D et al.. hiPSC-derived hepatocytes closely mimic the lipid profile of primary hepatocytes: A future personalised cell model for studying the lipid metabolism of the liver. J Cell Physiol. 2019;234 (4):3744-3761. doi: 10.1002/jcp.27131. PubMed PMID:30146765 .
- Paci, M, Pölönen, RP, Cori, D, Penttinen, K, Aalto-Setälä, K, Severi, S et al.. Automatic Optimization of an in Silico Model of Human iPSC Derived Cardiomyocytes Recapitulating Calcium Handling Abnormalities. Front Physiol. 2018;9 :709. doi: 10.3389/fphys.2018.00709. PubMed PMID:29997516 PubMed Central PMC6028769.
- Prajapati, C, Pölönen, RP, Aalto-Setälä, K. Simultaneous recordings of action potentials and calcium transients from human induced pluripotent stem cell derived cardiomyocytes. Biol Open. 2018;7 (7):. doi: 10.1242/bio.035030. PubMed PMID:29970475 PubMed Central PMC6078349.
- Juhola, M, Joutsijoki, H, Penttinen, K, Aalto-Setälä, K. Detection of genetic cardiac diseases by Ca2+transient profiles using machine learning methods. Sci Rep. 2018;8 (1):9355. doi: 10.1038/s41598-018-27695-5. PubMed PMID:29921843 PubMed Central PMC6008430.
- Mäki, AJ, Verho, J, Kreutzer, J, Ryynänen, T, Rajan, D, Pekkanen-Mattila, M et al.. A Portable Microscale Cell Culture System with Indirect Temperature Control. SLAS Technol. 2018;23 (6):566-579. doi: 10.1177/2472630318768710. PubMed PMID:29723086 .
- Strässler, ET, Aalto-Setälä, K, Kiamehr, M, Landmesser, U, Kränkel, N. Age Is Relative-Impact of Donor Age on Induced Pluripotent Stem Cell-Derived Cell Functionality. Front Cardiovasc Med. 2018;5 :4. doi: 10.3389/fcvm.2018.00004. PubMed PMID:29423397 PubMed Central PMC5790033.
- Prajapati, C, Ojala, M, Aalto-Setälä, K. Divergent effects of adrenaline in human induced pluripotent stem cell-derived cardiomyocytes obtained from hypertrophic cardiomyopathy. Dis Model Mech. 2018;11 (2):. doi: 10.1242/dmm.032896. PubMed PMID:29361520 PubMed Central PMC5894949.
2017
Kiamehr M, Viiri LE, Vihervaara T, Koistinen KM, Hilvo M, Ekroos K, Käkelä R, Aalto-Setälä K.
Lipidomic profiling of patient-specific iPSC-derived hepatocyte-like cells.
Dis Model Mech. 2017 Sep 1;10(9):1141-1153.
Kuusela J, Larsson K, Shah D, Prajapati C, Aalto-Setälä K.
Low extracellular potassium prolongs repolarization and evokes early afterdepolarization in human induced pluripotent stem cell-derived cardiomyocytes.
Biol Open. 2017 Jun 15;6(6):777-784. doi: 10.1242/bio.024216.
Vuorenpää H, Penttinen K, Heinonen T, Pekkanen-Mattila M, Sarkanen JR, Ylikomi T, Aalto-Setälä K.
Maturation of human pluripotent stem cell derived cardiomyocytes is improved in cardiovascular construct.
Cytotechnology. 2017 Apr 10. doi: 10.1007/s10616-017-0088-1.
2016
Laurila E, Ahola A, Hyttinen J, Aalto-Setälä K.
Methods for in vitro functional analysis of iPSC derived cardiomyocytes – Special focus on analyzing the mechanical beating behavior.
Biochim Biophys Acta. 2016 Jul;1863(7 Pt B):1864-72.
Ojala M, Prajapati C, Pölönen RP, Rajala K, Pekkanen-Mattila M, Rasku J, Larsson K, Aalto-Setälä K.
Mutation-Specific Phenotypes in hiPSC-Derived Cardiomyocytes Carrying Either Myosin-Binding Protein C Or α-Tropomyosin Mutation for Hypertrophic Cardiomyopathy.
Stem Cells Int. 2016;2016:1684792. doi:10.1155/2016/1684792
2015
Juhola M, Penttinen K, Joutsijoki H, Varpa K, Saarikoski J, Rasku J, Siirtola H, Iltanen K, Laurikkala J, Hyyrö H, Hyttinen J, Aalto-Setälä K
Signal analysis and classification methods for the calcium transient data of stem cell-derived cardiomyocytes
Comput Biol Med, 2015, 61:1–7
Kiviaho A, Ahola A, Larsson K, Kujala K, Pekkanen-Mattila M, Venäläinen H, Paavola K, Hyttinen J, Aalto-Setälä, K
Distinct electrophysiological and mechanical beating phenotypes of Long QT syndrome type 1 -specific cardiomyocytes carrying different mutations
IJC Heart & Vasculature, 2015, 8:19-31
Penttinen K, Swan H, Vanninen S, Paavola J, Lahtinen A-M, Kontula K, Aalto-Setälä K
Antiarrhythmic effects of Dantrolene in patients with catecholaminergic polymorphic ventricular tachycardia and replication of the responses using iPSC models
PLoS One, 2015, 10(5):e0135806
Penttinen K, Siirtola H, Ávalos-Salguero J, Vainio T, Juhola M, Aalto-Setälä K
Novel analysis software for detecting and classifying Ca2+ transient abnormalities in stem cell-derived cardiomyocytes
PLoS One, 2015, 10(8):e0135806
Kartasalo K, Pölönen R-P, Ojala M, Rasku J, Lekkala J, Aalto-Setälä K, Kallio P
CytoSpectre: a tool for spectral analysis of oriented structures on cellular and subcellular levels
BMC Bioinformatics, 2015, 16:344
Manzini, S, Viiri L, Marttila S, Aalto-Setälä K
A comparative view on easy to deploy non-integrating methods for patient-specific iPSC production
Stem Cell Rev, 2015, 11(6):900-908
2014
Ahola A, Kiviaho A, Larsson K, Honkanen M, Aalto-Setälä K, Hyttinen J
Video image-based analysis of single human induced pluripotent stem cell derived cardiomyocyte beating dynamics using digital image correlation
Biomed Eng Online, 2014, 13:39
2013
Toivonen S, Ojala M, Hyysalo A, Ilmarinen T, Rajala K, Pekkanen-Mattila M, Äänismaa R, Lundin K, Palgi P, Weltner J, Trokovic R, Silvennoinen O, Skottman H, Narkilahti S, Aalto-Setälä K, Otonkoski T
Comparative analysis of targeted differentiation of hiPSC and hESC reveals variability associated with incomplete transgene silencing in retrovirally derived hiPSC lines
Stem Cells Transl Med, 2013, 2(2):89-93
2012
Lahti A, Kujala V, Chapman H, Koivisto A-P, Pekkanen-Mattila M, Kerkelä E, Hyttinen J, Kontula K, Swan H, Conklin B, Yamanaka S, Silvennoinen O, Aalto-Setälä K
Model for long QT syndrome type 2 using human iPS cells demonstrates arrhythmogenic characteristics in cell culture
Dis Model Mech, 2012, 5(2):220-230
Contacts
Facility Director:
Katriina Aalto-Setälä, MD
katriina.aalto-setala(at)tuni.fi
Tel: +358 40 582 9567
Room: ARVO D437