Regulatory requirements for design and manufacture of medical devices
Up-to-date information on the requirements and regulations for different kind of medical devices in EU
Extent
5 ECTSCourse dates
Application period
Fees
Language of instruction
Study fields
Organising unit
Mode of study
Study level
Regulations on health technology products in Europe have changed significantly in recent years and many practices are still evolving. Up-to-date information on regulations and requirements are the keys to successful product development and manufacture of health technology products.
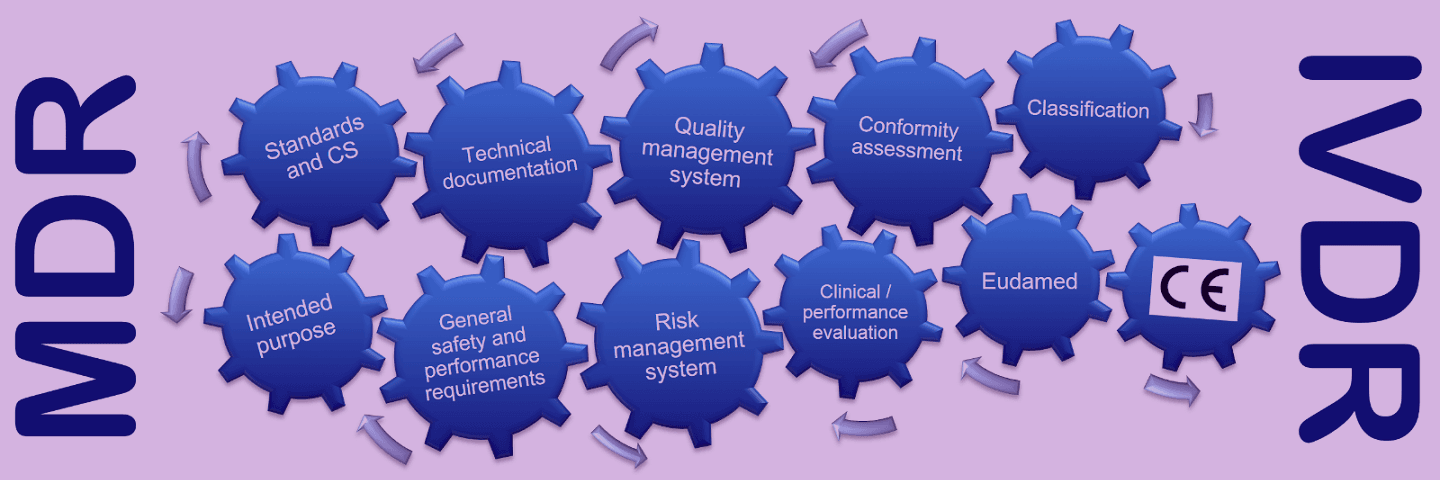
This course focuses on the EU Regulation on Medical Devices (MDR) and the EU Regulation on In Vitro Diagnostic Medical Devices (IVDR). In this course you will get up-to-date information e.g., general safety and performance requirements, classification, technical documentation, risk management system, clinical and performance evaluations, post market surveillance system, quality management system, conformity assessment and the role of conformity assessment bodies.
The course covers the following topics:
- Regulatory framework for medical devices in the European Union
- General safety and performance requirements for medical devices
- Classification of medical devices
- Technical documentation
- Risk management system (standard EN ISO 14971)
- Clinical evaluation and performance evaluation
- Post market surveillance system and vigilance
- Quality management system (standard EN ISO 13485)
- Conformity assessment procedures and the role of the conformity assessment bodies
- Registration requirements and European medical device database (EUDAMED)
After completing this course, you:
- are able to follow the constantly changing EU regulatory framework for medical devices
- are able to describe the regulatory pathway of a medical device from description of the intended purpose to placing into market and putting into service
- are able to recognise the general safety and performance requirements for a medical device including the principles of risk management and clinical evaluation (MD) or performance evaluation (IVD)
- are able to understand the main principles of a post-market surveillance system and challenges of vigilance system
- can explain the main elements of a quality system covering design and manufacture
- are able to select a conformity assessment procedure based on classification rules and chose a conformity assessment body
Teaching
The course includes lectures including theory and practice, joint discussions, and assignments. The course also includes product related assignments that can be applied to participants’ own organization. There is an exam at the end of the course.
Teaching is organized in Tampere at Hervanta campus, but it is possible to attend online as well. The course can be completed entirely online.
The teacher of the course is Petri Pommelin.
Petri Pommelin worked for the Finnish competent authority for medical devices from 1992 till 2006. Currently he is dealing with risk management and safety culture in health care.
Since 2005 he has regularly given lectures in a course on regulatory requirements for medical devices at Tampere University of Technology and Tampere University of Applied Sciences.
Target group
This course is targeted for those who are working or willing to work in health technology companies in different roles in R&D, quality and regulation, production, marketing and sales and management. The course gives essential knowledge also for health technology start-ups and anyone who wants to update his or her knowledge about MDR and IVDR.





