Scientists use light to control cell functions – future applications may turn sci-fi visions into reality

Previous research at the interface between photonics and biosciences has demonstrated that light-controlled smart materials are biologically compatible with cells. This is great news for tissue engineering as the findings help shed light on cell and tissue mechanics.
Disruptions in the biophysical activity of cells are associated with a number of diseases. The utilisation of light is a virtually unprecedented approach to the study of these cellular “power failures”.
“We use light-controlled surfaces to investigate how the topography of extracellular environment – meaning the lumps and bumps of the surface beneath the cells – and the dynamic changes occurring therein affect cell growth, movement and physiology. The findings may one day have important implications, for example, for cancer research” says Professor of Chemistry Arri Priimägi, who leads the Smart Photonic Materials (SPM) group at Tampere University.
Chiara Fedele, who completed a doctoral degree in materials engineering in Italy in 2017, has brought to the group her expert knowledge of combining light-controlled materials with cell and tissue engineering applications.
“Our multidisciplinary project brings together different research groups and creates a distinctly unique blend of expertise. Such an in-depth understanding of combining light-controlled materials with mechanobiology is rare even on a global scale,” Chiara Fedele notes.
“Chiara’s transition to Tampere, first as a visiting scientist and later as a post-doc, has played a pivotal role in our decision to explore this avenue of research. Without her input, it is unlikely that the SPM group would have embarked on this fascinating journey to the world of cells,” Priimägi says.
Smart materials to speed up wound healing in the future
The potential applications of light-controlled materials sound like something out of science fiction: it may one day be possible to integrate light-responsive components into tissues or develop mechanobiological engineered tissue transplants. But as Priimägi says, there is a long way to go before concrete applications become a reality.
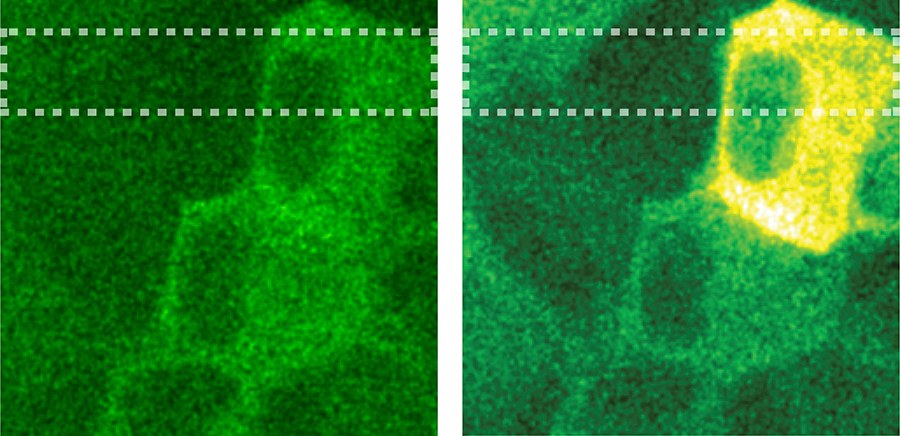
“Still, in a recently published study we confirmed that the movement and functions of epithelial cells, which resemble skin cells, can be controlled with the help of light-sensitive surfaces,” he adds.
Academy Research Fellow Teemu Ihalainen leads the Cellular Biophysics group, which also participates in the project. He is enthralled by the possibility to study the manipulation of cells at the interface between the living and the non-living.
“For a researcher, this is a veritable dream! Light can be used to precisely, repeatedly and effectively manipulate the tiny parts of a cell environment and monitor the response in real time,” Ihalainen points out.
In the future, light-controlled materials may be used, for example, to develop artificial blood vessels or muscles that are able to constrict, according to Ihalainen.
“A great deal of work remains to be done before these visions become a reality,” he emphasises.
University Researcher Soile Nymark leads the third member of the consortium, the Biophysics of the Eye group. She stresses the point that the consortium is undertaking basic research that increases our understanding of the potential of light and paves the way for practical applications.
“Basic research provides the foundation for applications,” Nymark notes.
The multidisciplinary consortium also includes doctoral researchers Mari Isomäki and Heidi Peussa and Academy Postdoctoral Researcher Elina Mäntylä. The research is funded by the Emil Aaltonen Foundation through the multidisciplinary ABioT project carried out by Tampere Universities.

The scientific article on the research Azobenzene-based sinusoidal surface topography drives focal adhesion confinement and guides collective migration of epithelial cells has been published on Nature.
Text: Anna Aatinen
Images: Jonne Renvall








